



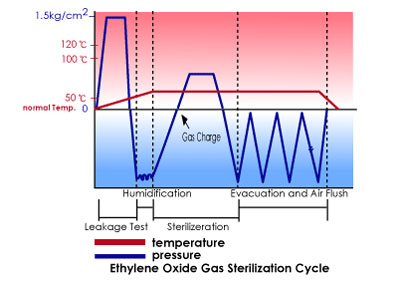
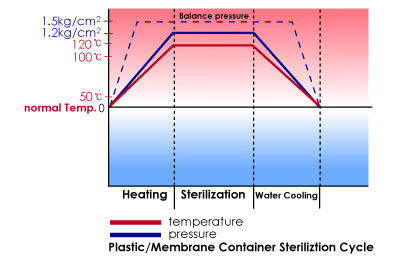
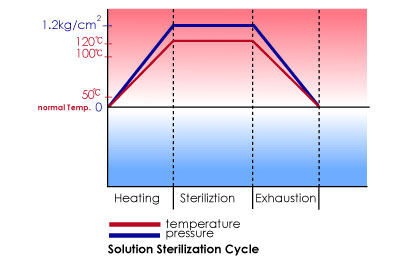
Our plant assure sterilization in conformity with good manufacturing practices for LVPs, SVP etc. and in accordance with the cGMP issued by FDA. in short, excellent engineering coupled with efficient manufacturing.

The autoclave was a central control panel, which houses the programmable logic controller (PLC), signal conditioning units, safety circuits and emergency switch, the autoclave is controlled at all time by the PLC. Which contains the operation automatic screen, manual trend graph, parameter, dynamic and password etc, screen.
Installation qualification (IQ)
Operational qualification (OQ)
Performance qualification (PQ)
|
Model
|
Capacity
|
Steam
Consuption |
Water
Consuption |
Electricity
Consuption |
Chamber
Dimension (WxHxD) |
External
Dimension (WxHxD) |
|
APSR-
200
|
200
|
55
|
30
|
1.5
|
500x500x800
|
1500x1800x1350
|
|
APSR-
668
|
288
|
60
|
30
|
1.7
|
600x600x800
|
1600x1950x1350
|
|
APSR-
400
|
400
|
100
|
40
|
2.3
|
500x800x1000
|
1500x2150x1750
|
|
APSR-
8108
|
640
|
120
|
40
|
2.8
|
800x800x1000
|
1800x2150x1750
|
|
APSR-
101010
|
1000
|
150
|
50
|
4
|
1000x1000x1000
|
2050x2300x1950
|
|
APSR-
101313
|
1780
|
160
|
60
|
5
|
1100x1300x1250
|
2150x2550x2300
|
|
APSR-
7200
|
7200
|
290
|
70
|
8.2
|
1200x2000x3000
|
2200x3300x3950
|
|
APSR-
500
|
177
|
140
|
50
|
1.5
|
500x900
|
600x1100
|
|
APSR-
1550
|
4720
|
180
|
60
|
5.2
|
1000x2600
|
1250x3200
|
|
APSR-
1800
|
15000
|
300
|
70
|
8.6
|
1800x6000
|
2050x7000
|